Shining a new light in the most challenging times
ReceptivaDx – a path forward for unexplained infertility, failed IVF, and recurrent pregnancy loss
Planning for a family and then dealing with infertility can be a heartbreaking journey, full of uncertainty and frustration. Many women experience failed IVF, recurrent pregnancy loss, or both. If you’ve gone through various fertility treatments without success, you might start to feel like you’re running out of options, especially when doctors can’t seem to find the answers.
That’s why we developed the ReceptivaDx test. This first-of-its-kind test is designed to help you get to the root of unexplained infertility, restoring your hope in the chances of a successful pregnancy.
What is ReceptivaDx?
ReceptivaDx is the only test that can identify leading causes of unexplained infertility in a single sample including endometriosis, progesterone resistance, and endometritis. ReceptivaDx includes BCL6, a marker that identifies uterine lining inflammation most often associated with asymptomatic (silent) endometriosis. BCL6 is found in more than 50% of women with unexplained infertility and around 65% of women with two or more IVF failures.
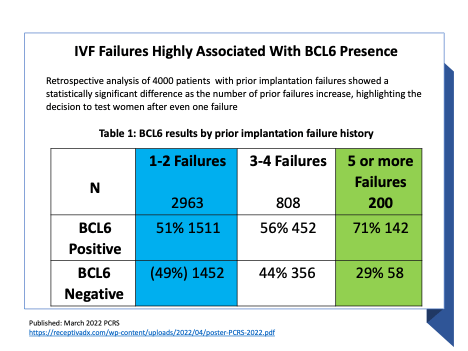
The ability to identify progesterone resistance via the BCL6 marker has been previously linked in published data to both implantation failure and recurrent pregnancy loss. Progesterone resistance is a condition that causes unfavorable changes on the uterine lining, blocking the important hormone progesterone from carrying out its vital role in embryo survival.
ReceptivaDx can also be used to identify endometritis, a chronic bacterial infection of the uterine lining, using a different marker called CD138. The good news for both providers and patients is that either a positive BCL6 or CD138 both have successful treatment options, significantly improving the chances for a successful pregnancy and live birth.

How do the test results help me?
Without treatment, women testing positive are 5 times less likely to succeed in IVF.
If positive for BCL6, current published data recommends two equally effective options:
- Surgical laparoscopy to remove any endometriosis.
- Hormone suppression therapy for 60 days which reduces the inflammation caused by endometriosis and provides a stable and viable surface for the embryo to grow.
If BCL6 is negative, provides assurance that uterine lining inflammation is not the primary cause of failed implantation and improves the probability of a successful transfer.
Useful Facts: Published studies show that women testing positive for BCL6 and left untreated have just an 11% chance of a successful live birth. Once treated, the probability goes up to over 60%. If negative, the probability is also above 60%.
Get Started
Get started and get answers. Find a center near you with our locator map. Questions? Call us for a free phone consultation. We’re here to help.
Learn More

ReceptivaDx: Helping With Unexplained Infertility

What Happens After You Experience IVF Failure?

Unexplained Infertility After My Miscarriage