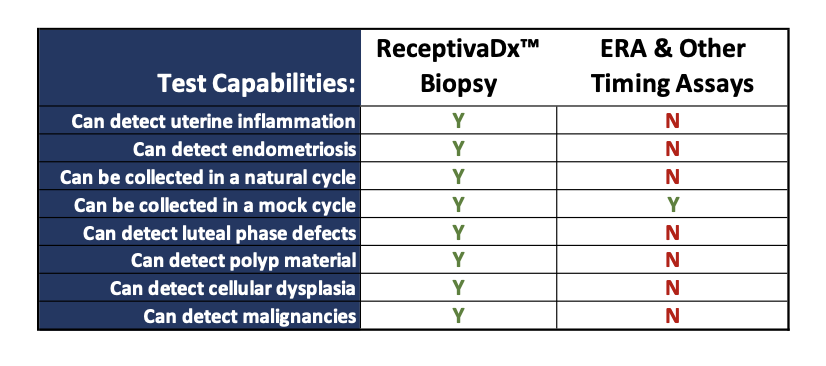
About ReceptivaDx
ReceptivaDx™ is an immunohistochemistry test that helps identify inflammatory conditions of the endometrium, most often associated with endometriosis, including silent endometriosis where no symptoms are obvious. The test provides enhanced screening for all stages of endometriosis and other conditions that interfere with implantation and endometrial receptivity. This is the first test for endometriosis with high sensitivity (93%) and specificity (96%). It is especially useful for women with unexplained infertility, IVF failure, or limited embryo reserves.
While other tests evaluate the quality of embryos or determine optimal embryo transfer windows, only ReceptivaDx can accurately detect inflammatory conditions on the uterine lining, the most common endometrial issue impacting fertility success. The results can help indicate what is likely causing implantation issues.
The test specifically identifies BCL6, a protein biomarker associated with inflammation. BCL6 is dramatically over-expressed in women with endometriosis, the most likely inflammatory condition causing implantation problems and unexplained infertility. Progesterone is required for a normal pregnancy, so a protein that interferes with progesterone action would explain much about the problems encountered in women with endometriosis.
Studies have shown that women with elevated BCL6 results and left untreated are five times less likely to succeed in IVF or subsequent transfer attempts. A negative BCL6 result provides reassurance to patients that endometriosis is not of concern. A December 2017 cohort study in Fertility and Sterility demonstrated the prognostic value of BCL6 results. Results were successful in predicting the likelihood of live birth on the next IVF transfer for patients experiencing unexplained infertility prior to the transfer. Since that early paper, over a dozen additional publications have come out highlighting the effectiveness of the test. Cicero Diagnostics has performed more than 20,000 tests and continues to collect and publish results on outcomes.
When to use ReceptivaDx
Recommendations on when to use our test clinically center around proven failure histories including recurrent pregnancy losses (miscarriages), but the test also should be considered in women with limited embryo reserves or limited financial means to cover multiple IVF cycles. Here are a few instances where the ReceptivaDx test would be appropriate:
Women with unexplained infertility
- Rule out uterine inflammation usually caused by endometriosis or endometritis (chronic bacterial infection)
- Establish chances of success or failure before beginning advanced fertility treatment options
Women with history of implantation failure
- Rule out uterine inflammation usually caused by endometriosis
- Rule out progesterone resistance, shown to be correlated with IVF failure
Women with recurrent pregnancy loss
- Rule out uterine inflammation usually caused by endometriosis
- Rule out progesterone resistance, shown to be correlated with recurrent pregnancy loss
Women biopsied for receptivity timing biopsies (ERA)
- Positive ReceptivaDx results indicate uterine lining dysfunction and/or progesterone resistance, obviating immediate concerns regarding optimized transfer timing
- Taking two biopsies in one procedure offers patient convenience and eliminates the need to schedule a future biopsy
Women with Limited Embryo Reserves
- Helps provide success probabilities upfront before the next transfer procedure regardless of the results of PGT testing
- Identifies potential progesterone resistance, reducing risk for recurrent pregnancy loss
Women with limited fertility coverage
- Helps better understand the potential causes of unexplained infertility
- Provides potential treatment pathways if IVF is not a financial option
- Helps women not pursuing advanced fertility assistance to potentially gain more information regarding recurrent pregnancy loss
Biopsy and Sample Collection for BCL6 and CD138
Natural Cycle: 7–10 days after ovulation
Mock Cycle: 6–10 days after start of progesterone
Biopsy Procedure
- Collect using standard pipelle
- Collect at the same time as other endometrial biopsies*
- Place in formalin vial only (provided in collection kit)
NOTE: Do not use vial provided by other companies. Many are not formalin - One pass/half pipelle is sufficient for analysis NOTE: Floating sample in vial indicates more mucosa than endometrial tissue therefore 2nd pass is recommended
- Preferred shipment days are Monday–Friday, although the sample is stable at room temperature for up to 14 days
*BCL6 analysis was not validated during egg retrieval cycles and therefore should not be obtained in that same cycle. Medications used during the egg retrieval cycle could impact results and do not represent an accurate portrayal of the uterine lining during an embryo transfer or natural cycle. Providers should wait until the next cycle to obtain an optimal biopsy.
Sample Submission
- Samples are collected in formalin only (provided in the collection kit) NOTE: Do not use vial provided by other companies. Many are not formalin
- Make sure the vial cap is secured (the cap clicking indicates the sample is secure)
- Place the vial in the hazard/safety bag (provided in the collection kit)
- Verify the two patient identifiers on the vial match the information on the test request form
- Verify the test request form is complete, including payment information and patient email
- Place the bag and test request form in the ReceptivaDx collection box
- Place collection box in FedEx Clinical Pak
- Attach the prepaid billable stamp
- Arrange for FedEx pick-up
Preferred shipment days are Monday–Friday FedEx overnight. Friday shipments will arrive on Monday. NOTE: The sample is stable at room temperature.
Results will be available 5–6 business days from the date received by the lab.
Results can only be faxed to the fax number provided on the test request form. Online access to the results is available. Please call 800-795-5385 to register your center.
Understanding Results
Both biomarkers BCL6 and CD138 are performed using Immunohistochemistry.
BCL6 is a protein marker highly associated with endometriosis. Results are reported as an H-score between 1-4. The H-score is calculated as the sum of the percentage of staining multiplied by an ordinal value corresponding to the intensity level and reported as (0 = none, 1 = weak, 2 = moderate, 3 = strong 4= very strong). Please note that the H-Score value is not an indicator of endometriosis disease stage.
The use of the test specific to infertility has been validated against laparoscopy-confirmed endometriosis patients using a cutoff value of 1.4. Any results above 1.4 should be considered positive and treated according to the guidelines under treatment. For clinical purposes, a value of 1.5 vs 4 will not affect protocols.
CD138 is an antigen specific to plasma cells and used to detect chronic endometritis. The results are calculated and reported based on presence of clusters of plasma cells. While rare stromal cells will be indicated, they are not considered consistent with a diagnosis of endometritis.
BCL6
- Positive results indicate inflammation of the uterine lining most likely caused by the body’s immune response to endometriosis. The test has been validated to be 93% sensitive and 96% specific for the presence of endometriosis. This inflammatory condition has proven to impact implantation and/or cause early pregnancy loss. Endometriosis is also associated with progesterone resistance thus negating even trigger shot therapies for many women.
- Left untreated, studies show patients with positive BCL6 have less than 12% chance for success on the next transfer cycle.
- A negative result indicates uterine lining is likely to be receptive.
CD 138
- Negative for endometritis: No cells staining positive for CD138
- Negative for endometritis: Some singular plasma cells are seen but insufficient for positive diagnosis.
- Positive for chronic endometritis: Multiple or clusters of cells seen staining positive.
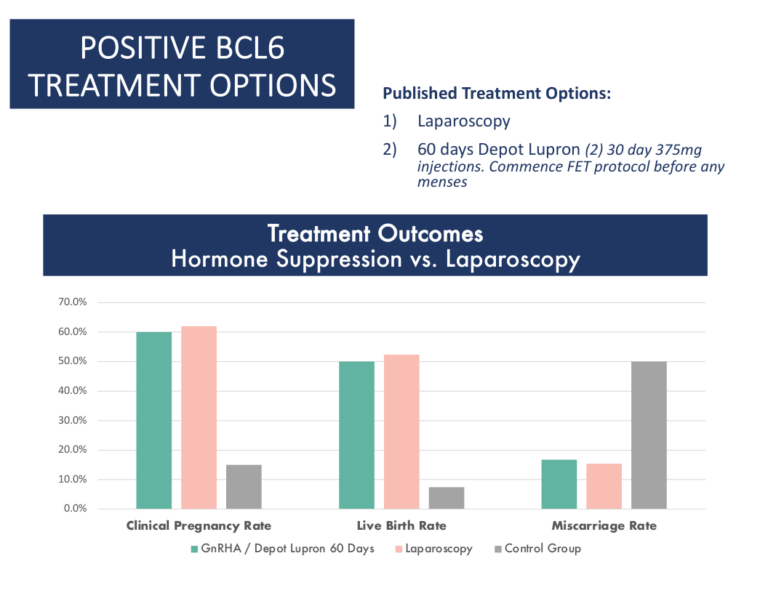
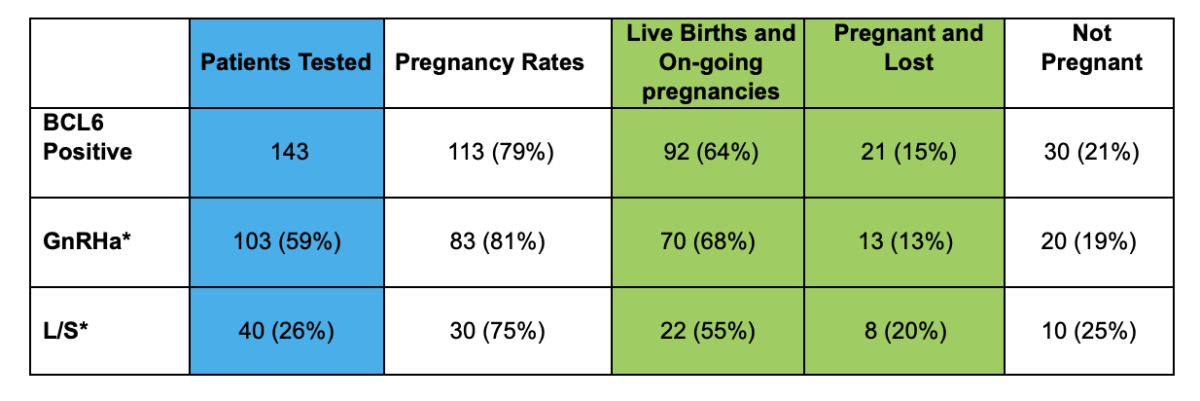
Treatment Options
Cicero Diagnostics cannot recommend specific treatments based on results of our assay. However, the published data and results of our outcomes studies continue to support the use of the treatment strategies below in the setting of IVF.
Treatment Strategies for a positive BCL6 Result
These fall into three categories:
- Laparoscopic surgery
- GnRh agonists such as Lupron (60 days)
- Letrozole
- As of 2020, clinical data supports both laparoscopic surgery or medical suppression using GnRh agonists as proven options for treatment of positive BCL6 patients. Source
- GnRh agonist suppression plus letrozole may be superior to GnRh agonist suppression alone. Source
- Treatment with letrozole alone during IVF has been shown to be effective in women with suspected endometriosis who lack Beta Integrin 3. Source
Treatment Strategies for a positive CD138 Result
A combination regimen of ampicillin, gentamicin, and metronidazole provides coverage against most of the organisms that are encountered in chronic endometritis. Doxycycline should be used if chlamydia is the cause of endometritis. Ampicllin sulbactam can be used as a monotherapy. Learn more
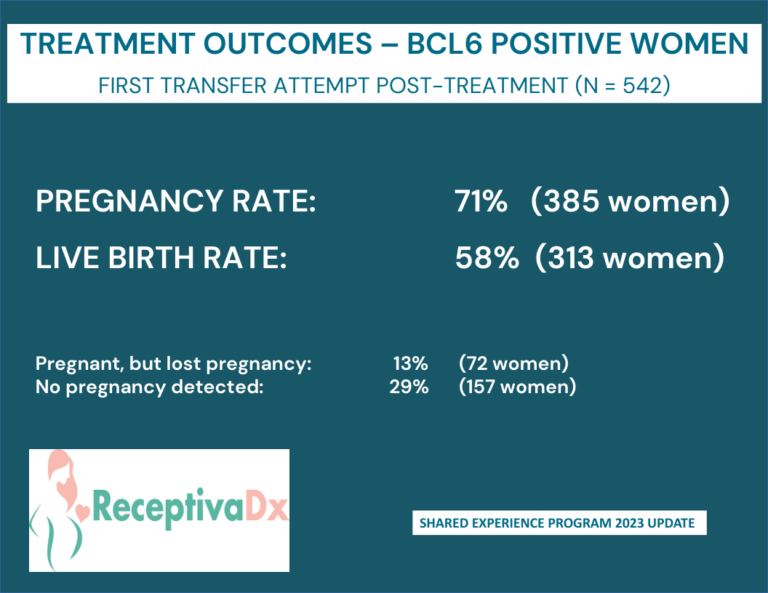
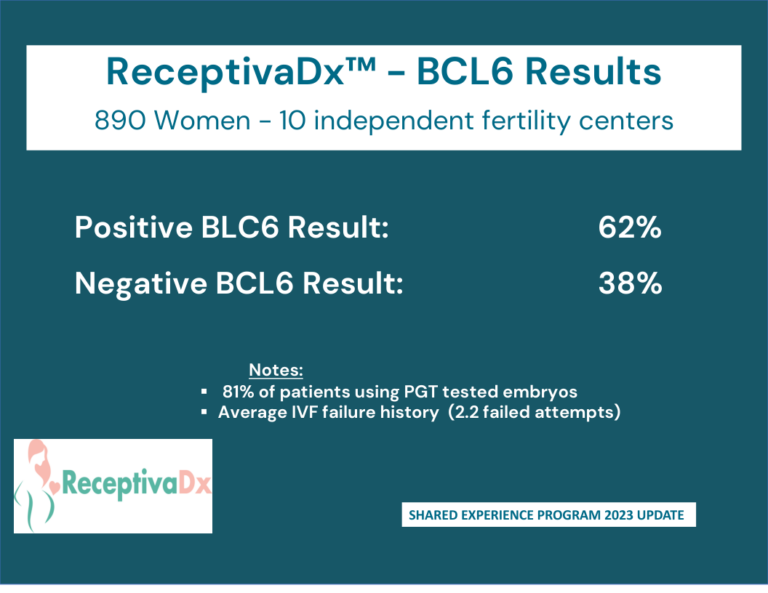
Clinical Data and Shared Experience
Clinical Data
Cicero Diagnostics is a data driven company dedicated to providing relevant and updated published clinical data to support the use of the ReceptivaDx test in the patient setting. As evidenced by the dozens of current independent studies as well as our own NIH supported research, Cicero Diagnostics provides more proven data than any other endometrial biopsy based test in the fertility sector.
Shared Experience Program
Since 2019, Cicero Diagnostics has offered a cooperative data analysis program with our IVF center clients providing centers a means to share their treatment and outcomes data on patients tested with ReceptivaDx. Participating centers with a minimum of 50 cases will have the data anonymously analyzed and compared to our aggregate outcomes database now exceeding 2,500 patients. Data from the Shared Experience Program is updated regularly and published in various formats. However, on an individual center level, we are able to dissect data to the specific needs of our clients.
For more information, contact Dan Angress at Dan@cicerodx.com
Frequently Asked Questions
Get Started
Call us at 800-795-5385 or send us an email at info@receptivadx.com for more information.